solution
solution.
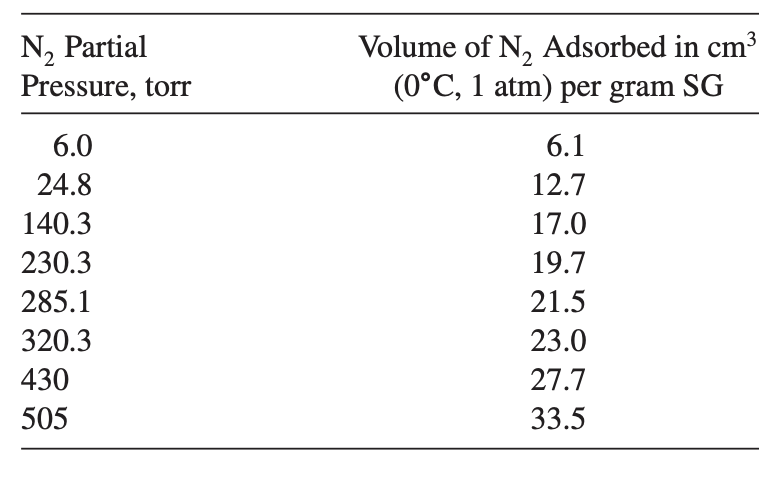
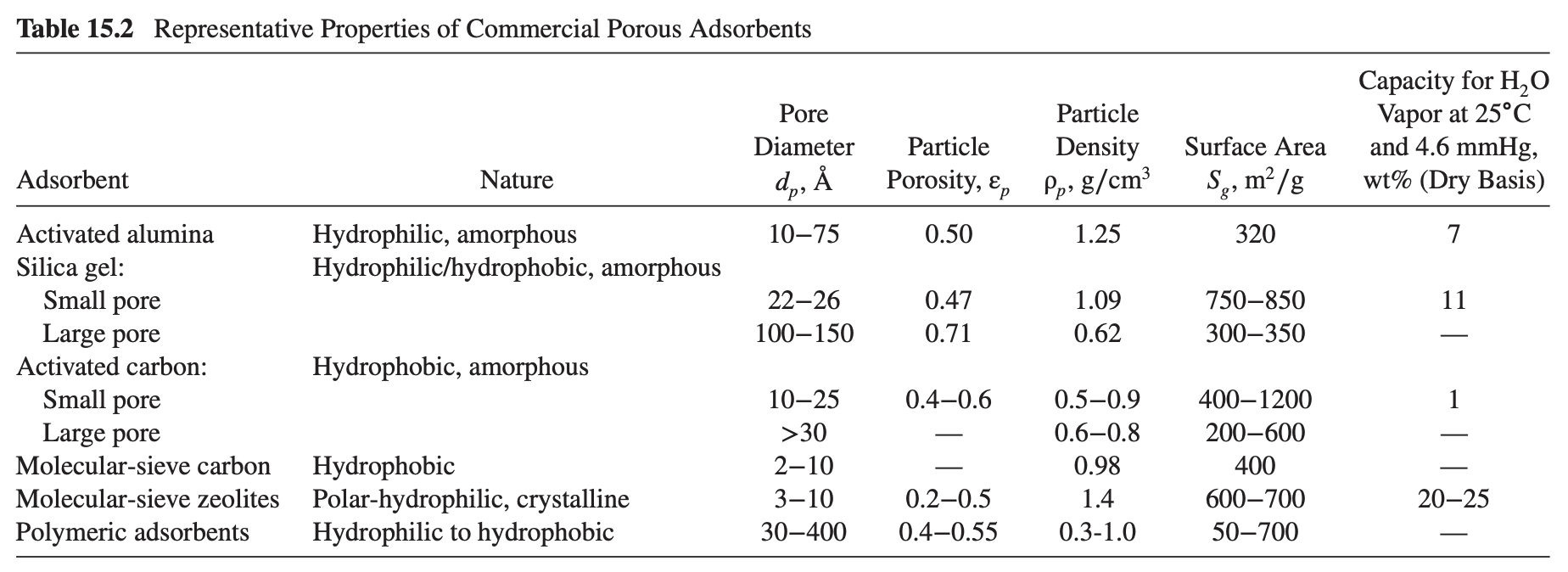
The following data were obtained in a BET apparatus for adsorption equilibrium of N2 on silica gel (SG) at -195.8°C. Estimate Sg in m2/g of silica gel. How does your value compare with that in Table 15.2?
Save your time - order a paper!
Get your paper written from scratch within the tight deadline. Our service is a reliable solution to all your troubles. Place an order on any task and we will take care of it. You wonŌĆÖt have to worry about the quality and deadlines
Order Paper NowN, Partial Pressure, torr Volume of N, Adsorbed in cm3 (0├é┬░C, 1 atm) per gram SG 6.0 24.8 140.3 230.3 285.1 320.3 430 505 6.1 12.7 17.0 19.7 21.5 23.0 27.7 33.5 Table 15.2 Representative Properties of Commercial Porous Adsorbents Capacity for H2O Vapor at 25├é┬░C and 4.6 mmHg, wt% (Dry Basis) Pore Diameter d├é┬╗, ├āŌĆ” 10├óŌé¼ŌĆ£75 Particle Density Pp, g/cm Particle Porosity, , Surface Area Sg, m├é┬▓/g Adsorbent Nature 0.50 1.25 320 Hydrophilic, amorphous Hydrophilic/hydrophobic, amorphous 22├óŌé¼ŌĆ£26 100├óŌé¼ŌĆ£150 0.47 0.71 1.09 0.62 750├óŌé¼ŌĆ£850 300-350 11 Hydrophobic, amorphous Activated alumina Silica gel: Small pore Large pore Activated carbon: Small pore Large pore Molecular-sieve carbon Molecular-sieve zeolites Polymeric adsorbents 0.4-0.6 Hydrophobic Polar-hydrophilic, crystalline Hydrophilic to hydrophobic 10├óŌé¼ŌĆ£25 >30 2├óŌé¼ŌĆ£10 3├óŌé¼ŌĆ£10 30├óŌé¼ŌĆ£400 – 0.2-0.5 0.4-0.55 0.5-0.9 0.6-0.8 0.98 1.4 0.3-1.0 400├óŌé¼ŌĆ£1200 200-600 400 600├óŌé¼ŌĆ£700 50├óŌé¼ŌĆ£700 20├óŌé¼ŌĆ£25
“Looking for a Similar Assignment? Get Expert Help at an Amazing Discount!”

